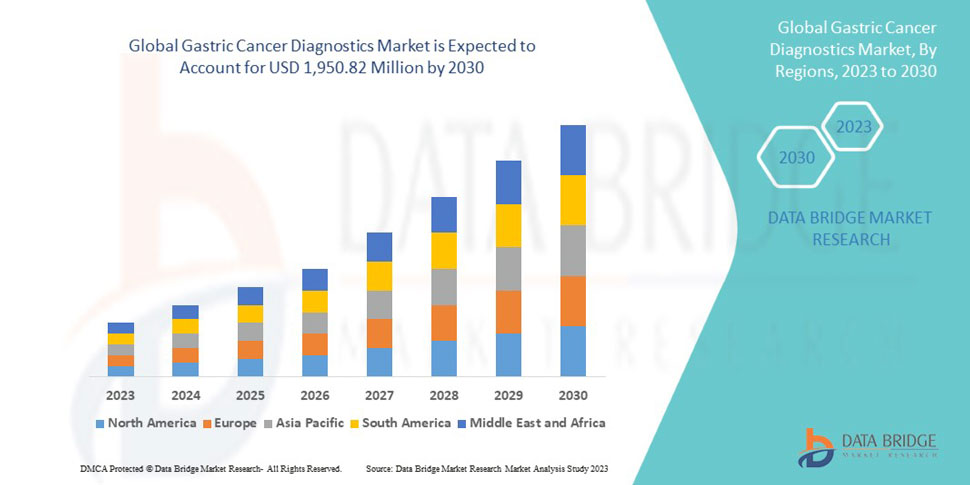
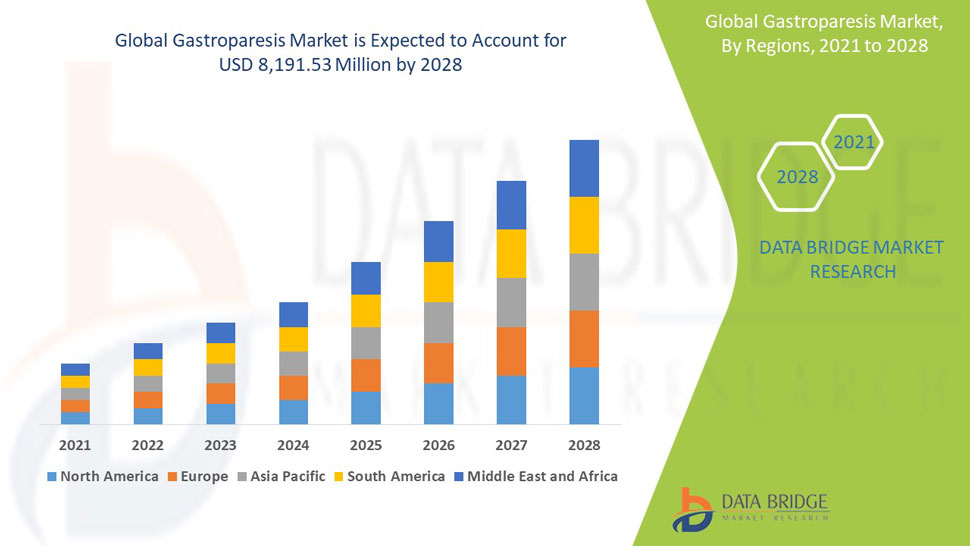
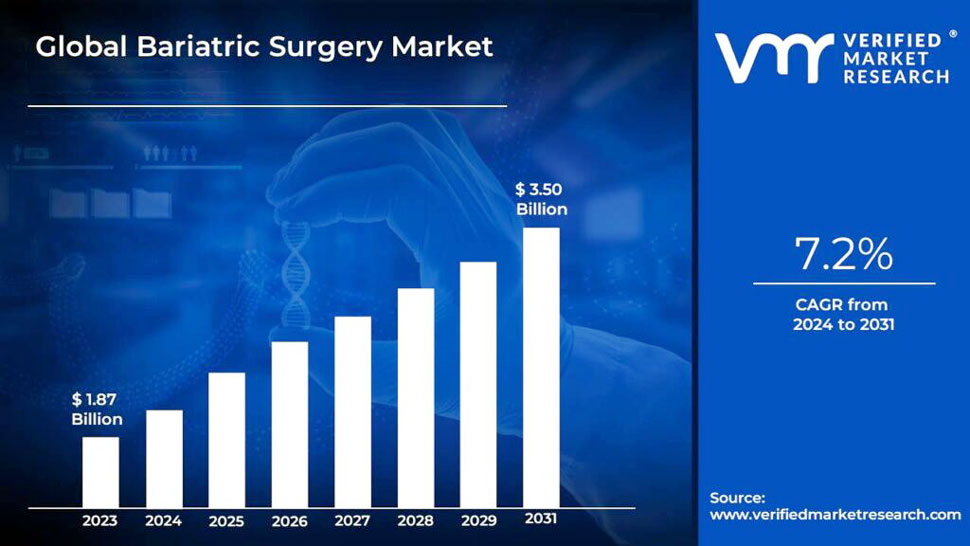
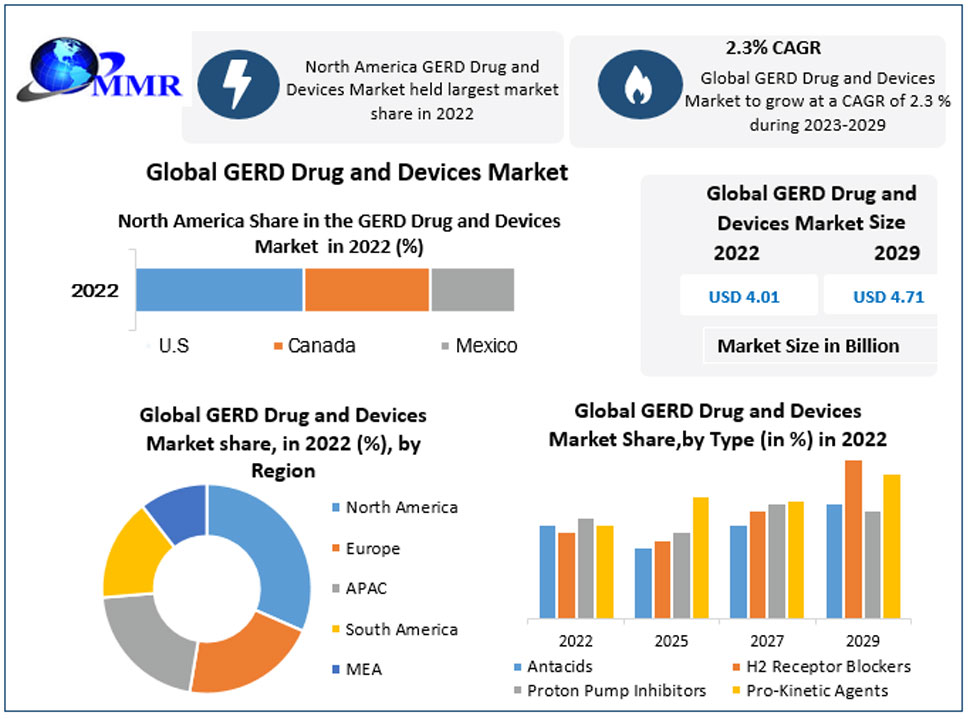
Endo-TAGSS is in the process of obtaining regulatory approval. Medical conditions and procedures the TAGSS device will have the capacity to assist in addressing include bariatric surgery, gastroparesis, fundoplication to treat Gastroesophageal Reflux Disease (GERD), myotomy for achalasia treatment, esponge placement for care of perforated esophagus, treatment of refractory upper gastrointestinal bleeding, and gastric tumor removal. Endo-TAGSS is currently raising a $2.1 million round of funding. This email address is being protected from spambots. You need JavaScript enabled to view it. for more information.